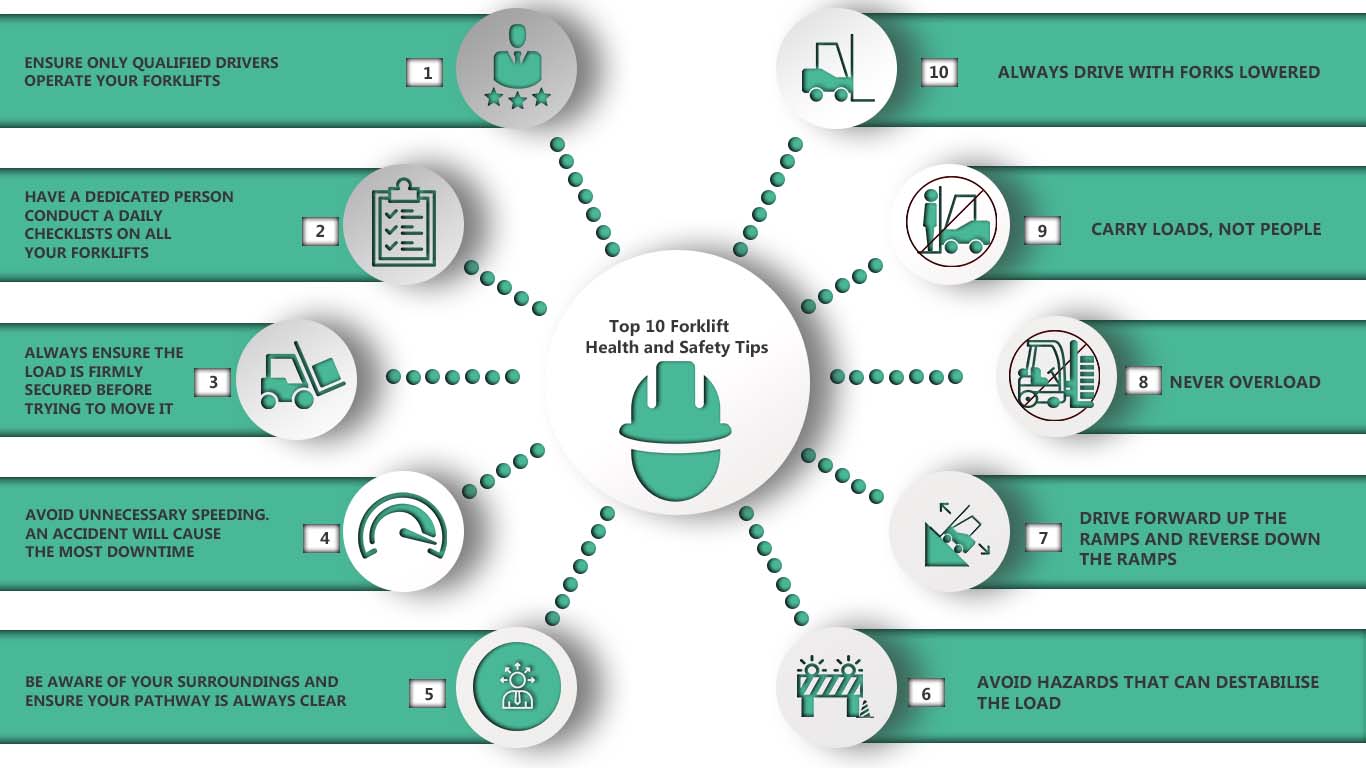
Q:What is the Meaning of LEL , UEL and PID ?
A: Lower and Upper Explosive Limits for Flammable Gases and Vapors
Before a fire or explosion can occur, three conditions must be met simultaneously.
A fuel (ie. combustible gas) and oxygen (air) must exist in certain proportions, along with an ignition source, such as a spark or flame. The ratio of fuel and oxygen that is required varies with each combustible gas or vapor.
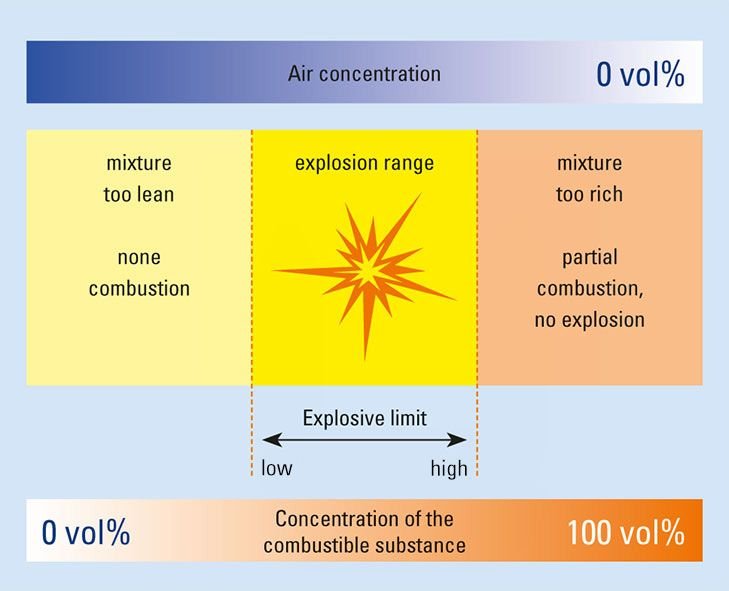
The minimum concentration of a particular combustible gas or vapor necessary to support its combustion in air is defined as the Lower Explosive Limit (LEL) for that gas. Below this level, the mixture is too “lean” to burn. The maximum concentration of a gas or vapor that will burn in air is defined as the Upper Explosive Limit (UEL). Above this level, the mixture is too “rich” to burn. The range between the LEL and UEL is known as the flammable range for that gas or vapor.

Lower and Upper Explosive Limits
The values shown in the table below are valid only for the conditions under which they were determined (usually room temperature and atmospheric pressure using a 2 inch tube with spark ignition). The flammability range of most materials expands as temperature, pressure and container diameter increase. All concentrations in percent by volume.
| Gas | LEL | UEL |
| Acetone | 2.6 | 13 |
| Acetylene | 2.5 | 100 |
| Acrylonitrile | 3 | 17 |
| Allene | 1.5 | 11.5 |
| Ammonia | 15 | 28 |
| Benzene | 1.3 | 7.9 |
| 1.3-Butadiene | 2 | 12 |
| Butane | 1.8 | 8.4 |
| n-Butanol | 1.7 | 12 |
| 1-Butene | 1.6 | 10 |
| Cis-2-Butene | 1.7 | 9.7 |
| Trans-2-Butene | 1.7 | 9.7 |
| Butyl Acetate | 1.4 | 8 |
| Carbon Monoxide | 12.5 | 74 |
| Carbonyl Sulfide | 12 | 29 |
| Chlorotrifluoroethylene | 8.4 | 38.7 |
| Cumene | 0.9 | 6.5 |
| Cyanogen | 6.6 | 32 |
| Cyclohexane | 1.3 | 7.8 |
| Cyclopropane | 2.4 | 10.4 |
| Deuterium | 4.9 | 75 |
| Diborane | 0.8 | 88 |
| Dichlorosilane | 4.1 | 98.8 |
| Diethylbenzene | 0.8 | |
| 1.1-Difluoro-1-Chloroethane | 9 | 14.8 |
| 1.1-Difluoroethane | 5.1 | 17.1 |
| 1.1-Difluoroethylene | 5.5 | 21.3 |
| Dimethylamine | 2.8 | 14.4 |
| Dimethyl Ether | 3.4 | 27 |
| 2.2-Dimethylpropane | 1.4 | 7.5 |
| Ethane | 3 | 12.4 |
| Ethanol | 3.3 | 19 |
| Ethyl Acetate | 2.2 | 11 |
| Ethyl Benzene | 1 | 6.7 |
| Ethyl Chloride | 3.8 | 15.4 |
| Ethylene | 2.7 | 36 |
| Ethylene Oxide | 3.6 | 100 |
| Gasoline | 1.2 | 7.1 |
| Gas | LEL | UEL |
| Gas | LEL | UEL |
| Heptane | 1.1 | 6.7 |
| Hexane | 1.2 | 7.4 |
| Hydrogen | 4 | 75 |
| Hydrogen Cyanide | 5.6 | 40 |
| Hydrogen Sulfide | 4 | 44 |
| Isobutane | 1.8 | 8.4 |
| Isobutylene | 1.8 | 9.6 |
| Isopropanol | 2.2 | |
| Methane | 5 | 17 |
| Methanol | 6.7 | 36 |
| Methylacetylene | 1.7 | 11.7 |
| Methyl Bromide | 10 | 15 |
| 3-Methyl-1-Butene | 1.5 | 9.1 |
| Methyl Cellosolve | 2.5 | 20 |
| Methyl Chloride | 7 | 17.4 |
| Methyl Ethyl Ketone | 1.9 | 10 |
| Methyl Mercaptan | 3.9 | 21.8 |
| Methyl Vinyl Ether | 2.6 | 39 |
| Monoethylamine | 3.5 | 14 |
| Monomethylamine | 4.9 | 20.7 |
| Nickel Carbonyl | 2 | |
| Pentane | 1.4 | 7.8 |
| Picoline | 1.4 | |
| Propane | 2.1 | 9.5 |
| Propylene | 2.4 | 11 |
| Propylene Oxide | 2.8 | 37 |
| Styrene | 1.1 | |
| Tetrafluoroethylene | 4 | 43 |
| Tetrahydrofuran | 2 | |
| Toluene | 1.2 | 7.1 |
| Trichloroethylene | 12 | 40 |
| Trimethylamine | 2 | 12 |
| Turpentine | 0.7 | |
| Vinyl Acetate | 2.6 | |
| Vinyl Bromide | 9 | 14 |
| Vinyl Chloride | 4 | 22 |
| Vinyl Fluoride | 2.6 | 21.7 |
| Xylene | 1.1 | 6.6 |
| Gas | LEL | UEL |
Principles of Gas Detection
One of the many requirements for entering confined spaces is the measurement for flammable gases. Prior to entry of a confined space, the level of flammable gases must be below 10% of LEL.
The most common sensor used for measuring LEL is the Wheatstone bridge/catalytic bead/pellistor sensor (“Wheatstone bridge”).
LEL Sensors Explained
A Wheatstone bridge LEL sensor is simply a tiny electric stove with two burner elements. One element has a catalyst (such as platinum) and one doesn’t. Both elements are heated to a temperature that normally would not support combustion.
However, the element with the catalyst “burns” gas at a low level and heats up relative to the element without the catalyst. The hotter element has more resistance and the Wheatstone bridge measures the difference in resistance between the two elements, which correlates to LEL.

Unfortunately, Wheatstone bridge sensors fail to an unsafe state; when they fail, they indicate safe levels of flammable gases. Failure and/or poisoning of Wheatstone bridge LEL sensor can only be determined through challenging Wheatstone bridge sensors with calibration gas.
LEL Sensors Limitations
Two mechanisms affect the performance of Wheatstone bridge LEL sensors and reduce their effectiveness when applied to all but methane:
- GASES BURN WITH DIFFERENT HEAT OUTPUTS
Some gases burn hot and some burn relatively cool. These differing physical characteristics lead to difficulties when using LEL sensors. For example, 100% of LEL Methane (5% methane by volume) burns with twice the heat of 100% of LEL Propane (2.0 propane by volume). - HEAVIER HYDROCARBON VAPORS HAVE DIFFICULTY DIFFUSING INTO LEL SENSORS AND REDUCE THEIR OUTPUT
Some Heavier hydrocarbon vapors have difficulty diffusing through the sintered metal flame arrestor on LEL sensors. This flame arrestor is necessary to prevent the sensor itself from starting a fire and does not prevent gases like methane, propane and ethane from reaching the Wheatstone bridge. However, hydrocarbons like gasoline, diesel, solvents, etc, diffuse through the flame arrestor slower so that less vapor reaches the Wheatstone bridge and the sensor gives less output.
Why Not Use an LEL Monitor?
Many Volatile Organic Compounds (VOCs) are flammable and may be detected by the LEL or combustible gas sensors found in virtually every multigas monitor. However, LEL sensors are not particularly useful in measuring toxicity because they do not have enough sensitivity.
WHAT ARE SOME COMMON VOCS?
VOCs are the chemical compounds that keep industry going and include:
- Fuels
- Oils, °reasers, Heat Transfer Fluids
- Solvents, Paints
- Plastics, Resins and their precursors
- and many others
VOCs are found throughout industry, from the obvious applications in the petro-chem industry to not-so-obvious applications such as sausage manufacturing.
LEL Sensors Measure Explosivity, Not Toxicity
LEL sensors measure percent of LEL. For example, Gasoline has an LEL of 1.4%. Therefore, 100% of LEL is 14,000 ppm of gasoline, 10% of LEL is 1,400 ppm of gasoline and 1% of LEL is 140 ppm of gasoline.
140 ppm of gasoline is the lowest amount of vapor that the LEL monitor can “see.” Gasoline has a TWA of 300 ppm and a STEL of 500 ppm; this does not make LEL sensors well suited for measuring gasoline vapors because they simply don’t provide adequate resolution.
LEL sensors measure explosivity, not toxicity. Many VOCs are potentially toxic at levels that are well below their explosive levels and below the sensitivity of the LEL sensors.
AS DESCRIBED ABOVE:
One of the many requirements for entering confined spaces called is the measurement of confined spaces for flammable gases.
Prior to entry of a confined space, the level of flammable gases must be below 10% of LEL.
The most common sensor used for measuring LEL is the Wheatstone bridge/catalytic bead/pellistor sensor (“Wheatstone bridge”).
While useful in a wide variety of applications, in some settings Wheatstone bridge LEL sensors either don’t have enough sensitivity to a particular chemical, or chemicals used in the environment can render the Wheatstone bridge sensor inoperable.
In these types of circumstances, PIDs (photoionization detectors) can provide an alternative, highly accurate, and poison-free means of measuring 10% of LEL for confined space entry.
What is a PID?
A Photo-Ionization Detector measures VOCs and other toxic gases in low concentrations from ppb (parts per billion) up to 10,000 ppm (parts per million or 1% by volume).
A PID is a very sensitive broad-spectrum monitor, like a “low-level LEL monitor. A Photo-Ionization Detector measures VOCs and other toxic gases in low concentrations from ppb (parts per billion) up to 10,000 ppm (parts per million or 1% by volume). A PID is a very sensitive broad-spectrum monitor, like a “low-level LEL monitor.
How does a PID Work?
A Photo Ionization Detector (PID) uses an Ultraviolet (UV) light source (Photo= light) to break down chemicals to positive and negative ions (Ionization) that can easily be counted with a Detector. Ionization occurs when a molecule absorbs the high energy UV light, which excites the molecule and results in the temporary loss of a negatively charged electron and the formation of positively charged ion.

The gas becomes electrically charged. In the Detector these charged particles produce a current that is then amplified and displayed on the meter as “ppm” (parts per million) or even in “ppb” (parts per billion).
The ions quickly recombine after the electrodes in the detector to “reform” their original molecule.
PIDs are non-destructive; they do not “burn” or permanently alter the sample gas, which allows them to be used for sample gathering.
What does a PID measure?
The largest group of compounds measured by a PID are the Organics: compounds containing Carbon (C) atoms. These include:

- Aromatics – compounds containing a benzene ring including benzene, toluene, ethyl benzene and xylene
- Ketones and Aldehydes – compounds with a C=O bond including acetone, methyl ethyl ketone (MEK) and acetaldehyde
- Amines and Amides – Carbon compounds containing nitrogen, like diethylamine
- Chlorinated hydrocarbons – trichloroethylene (TCE), perchloroethylene (PERC)
- Sulfur compounds – mercaptans, sulfides
- Unsaturated hydrocarbons – like butadiene and isobutylene
- Alcohol’s- like isopropanol (IPA) and ethanol
- Saturated hydrocarbons – like butane and octane. In addition to organic compounds, PIDs can be used to measure some Inorganics. These are compounds without carbon and include:
- Ammonia
- Semiconductor gases: Arsine, Phosphine
- Hydrogen sulfide
- Nitric Oxide
- Bromine and Iodine