As an employer of health care workers, you want and need to provide a safe and healthy workplace for your employees. In 1991, OSHA published the Bloodborne Pathogens Standard, Title 29 Code of Federal Regulations, Part 1910.1030, to protect workers from exposures to bloodborne illnesses. Because needlestick injuries are a major cause of these exposures in the health care setting, it is important to recognize that there are work practices and engineering controls to help reduce these exposures and injuries. This brochure looks at the issue of safer needle devices and how they can help employers like you create a safer workplace to protect your workers.
Why Do I Need to Worry About Needlesticks?
If you’re an employer of health care workers who are potentially exposed to blood and contaminated needles, you should know that there are an estimated 800,000 needlesticks each year in the U.S. About 2 percent, or 16,000, of these, are likely to be contaminated with the Human Immunodeficiency Virus (HIV). Needlestick injuries account for up to 80 percent of accidental exposures to blood. Nurses in hospitals are the most frequently injured.
When Might My Employees Be Injured By a Needlestick?
Needlestick injuries may occur when employees dispose of needles, collect and dispose of materials used during patient care procedures, administer injections, draw blood, or handle trash or dirty linens where needles have been inappropriately discarded.
Isn’t There Just a Small Chance of Such an Injury?
Data from 63 hospitals show that the overall rate of such injuries is 27 per 100 occupied beds annually. Nurses
had the most frequent
exposures (49.7 percent); physicians ranked second (12.6 percent); nursing assistants accounted for 5.3 percent, and housekeepers, 5.1 percent. Hollow-bore needles are the cause of injury in 68.5 percent of all cases.
What Can Happen from a Needlestick?
More than 20 pathogens have been reportedly transmitted from needlesticks. The most serious is the transmission of Hepatitis C(HCV), Hepatitis B (HBV), and HIV. In fact, the risk of acquiring HBV or HCV from a contaminated needlestick is greater than for HIV.
(Learn More: HIV -AIDS-exposure )
Why Is the Risk Greater from Hepatitis B and C Than from HIV?
The risk of acquiring an infection has to do with the prevalence of these diseases in the patient population at large. For example, an estimated 1.25 million people in the U.S. are chronically infected with HBV, and 6,000 die each year from HBV-related liver disease. HCV also is a major cause of chronic liver disease worldwide. In 1997, there were an estimated 4 million people in the U.S. infected with HCV. As many as 85 percents of all HCV infected persons develop chronic hepatitis and are at increased risk for cirrhosis and primary hepatocellular carcinoma. Liver failure from Hepatitis C is the leading reason for liver transplants in the U.S.
(Read more: Hepatitis B and C Risks )
So, Do I Still Need to Worry About HIV Exposures for Employees?
Yes. The total number of occupationally acquired HIV infections in health care workers continues to increase each year. Of the 52 such cases documented during 1996, 45 were from needlesticks or cuts.
How Can I Protect EmployeesAgainst Potential Exposures?
Make sure that employees use universal precautions, engineering, and work practice controls, and personal protective equipment to reduce their exposure to bloodborne pathogens, as required by OSHA’s Bloodborne Pathogens Standard.
Can’t Needles Penetrate Most Personal Protective Equipment?
You’re correct. Most personal protective equipment can be easily penetrated by needles. Most needlestick injuries, however, result from unsafe needle devices rather than carelessness by health care workers. Safer needle devices have been shown to significantly reduce needlesticks and exposures to potentially fatal bloodborne illnesses.
What’s a Safer Needle Device?
A safer needle device has built-in safety controls to reduce needlestick injuries before, during, or after use, and to make needlesticks less likely.
Will These Devices Prevent Needlestick Injuries?
Not all needlestick injuries are preventable, but the number can be reduced by using devices containing needles with built-in safety features or other devices that eliminate the use of needles altogether. Using needleless IV connectors, self re-sheathing needles, or blunted surgical needles, for example, can help reduce the risk of injury. In fact, almost 83 percent of injuries from hollow bore needles are potentially preventable.
How Do These Devices Work?
In general, properly designed devices should (1) provide a barrier between the hands and the needle after use; (2) allow or require the worker’s hands to remain behind the needle at all times; (3) have safety features integral to the device itself rather than as accessories; (4) be in effect before disassembly and remain in effect after disposal to protect downstream workers; (5) be simple and easy to operate, with little or no training; and (6) not interfere with the delivery of patient care.
Are There Specific Safety Features I Need to Know About?
Yes, that would be helpful. For example, it is good to know whether the feature is active or passive or whether the engineering control is part of the device. Types of safety features include the following:
- Passive safety features remain in effect before, during, and after use; workers do not have to activate them. Passive features enhance safety design and are more likely to have a greater impact on prevention. (See Figure 1.)
- Active devices require the worker to activate the safety mechanism. Failure to do so leaves the worker unprotected. Proper use by health care workers is the primary factor in the effectiveness of these devices. (See Figure 2.)
- An integrated safety design means that the safety feature is built-in as an integral part of the device and cannot be removed. This design feature is usually preferred. (See Figure 1.)
- An accessory safety device is a safety feature that is external to the device and must be carried to, or be temporarily or permanently fixed to, the point of use. This design also is dependent on employee compliance, and according to some researchers, is less desirable.
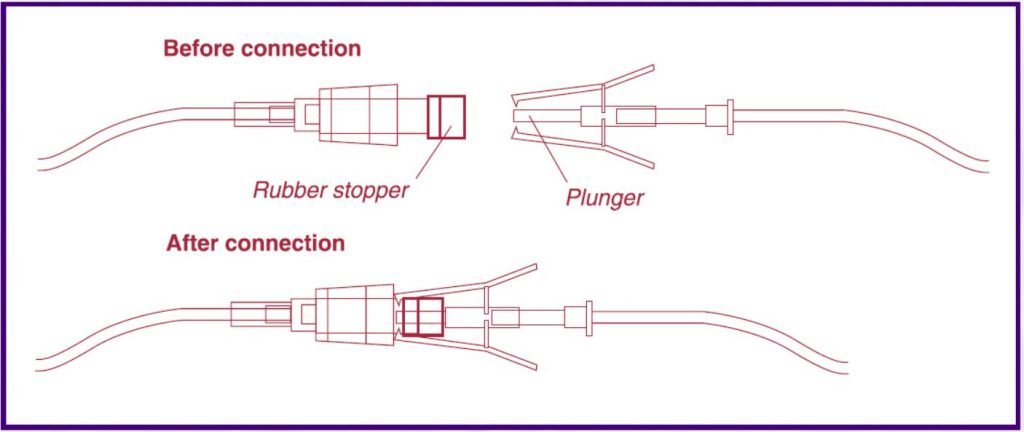
These connectors use devices other than needles to connect one IV to another. Examples of needleless connectors include 3-way stopcocks and plunger-type systems. An example of the plunger-type system is shown here. These devices are passive and integral to the system.
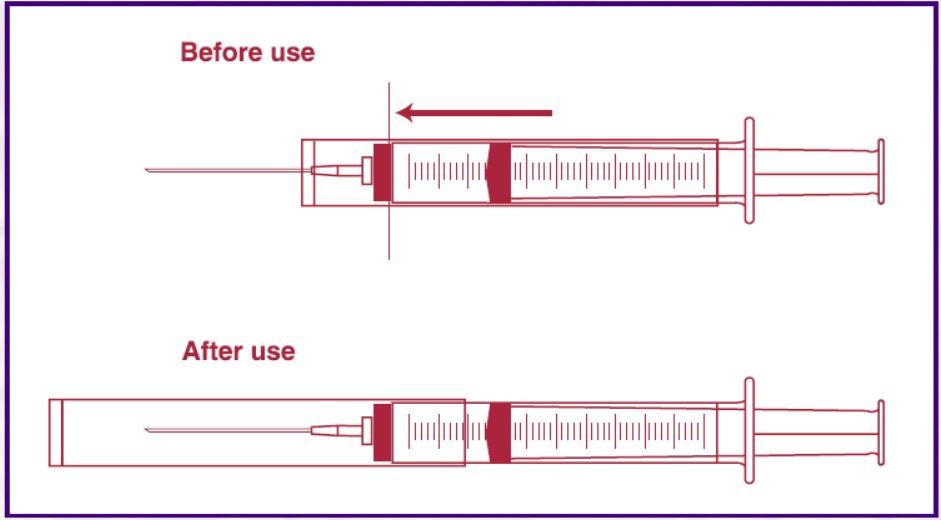
Initially, the sleeve is located over the barrel of the syringe with the needle exposed for use. After the device is used, the user slides the sleeve forward over the needle where it locks in place and provides a guard around the used needle.
Does OSHA Require the Use of Specific Devices?
No. OSHA does not require employers to institute specific devices, but it does require that employers evaluate the effectiveness of existing controls and review the feasibility of instituting more advanced engineering controls. Further, OSHA’s Bloodborne Pathogens Standard requires that employers establish a written exposure control plan as well as engineering and work practice controls to eliminate or minimize employee exposure. Additionally, employers are required to provide post-exposure follow-up if an employee sustains a needle puncture and to record the injury on the OSHA 200 log in some cases.
What Steps Do I Need to Take to Have a Comprehensive Prevention Program and to Implement Safer Needle Devices?
As an employer of health care workers, you have the flexibility to develop individual worksite-specific needlestick prevention programs to protect employees. Such a program would mean that you have a mechanism in place to select and evaluate safer medical devices in a systematic manner. In evaluating safer needlestick devices, ideally, you should evaluate your workplace and base your choices for these types of products on the following:
- The needs of the primary users.
- The need of the patients who must continue to receive safe, efficient, and comfortable care. (Workers are likely to reject products that they think will interfere with patient care in any way.)
In addition, a comprehensive needlestick prevention program might include the following:
- Creating a multidisciplinary team to investigate and assess needlestick incidents.
- Defining prevention priorities on the basis of collection and analysis of an institution’s injury data.
- Developing design and performance criteria for product selection according to needs for patient care and health care worker safety.
- Planning and implementing an evaluation of products in clinical settings.
(Read more:templates-bloodborne-pathogens-and-bbp-exposure-control-plan/ )
To evaluate and select appropriate safer needle devices, you also should review available needlestick injury data including the personnel involved, the devices used, and the circumstances and frequency of needlestick events. This information can help in determining how employees can maximally benefit from a product change to safer needle devices. Although not required by OSHA, the collection and evaluation of complete needlestick injury data are key to identifying injury patterns and then implementing an effective abatement plan. (See also, the sample “Safety Feature Evaluation Form,” for help in determining the most appropriate device for your employees.)

Download the Safety Feature Evaluation Form Guideline from here
Video Source :
Preventing Needle Stick
Struck by Needle